

|
|
|
ORGANIC CONDUCTORS" Charge Transfer Complexes "Written by Fraboni Andrea |
|
Usually we are induced to consider organic compounds as insulators. Indeed, also organic compounds can conduct. How that occurs is following explained. We know that all substances may be classified electrically as conductors or insulators, according to the degree of resistance, which the medium offers to the flow of current. It is usual to consider electric current as a flow of electrons from one point to another through a medium. Both electrons and ions can be seen as charge carriers responsible of the electric conductivity inside the solids. The metals are good electric conductors because they are in presence of a partial overlap between the conduction band and the valence band, allowing the electrons moves freely through the metallic solid. The insulators are bad electric conductors because they are in presence of a great energy gap between the conduction band and the valence band than they cannot be conductors. When, we are in presence of semiconductors, the energy gap between the valence band and the conduction band is not excessive and we can overcome the gap by two manners:
If we going to dope Silicon with Boron or Arsenic respectively to produce the doping type-P (electron vacancy) and doping type-N (electron excess) we have realized a good electric conductor at room temperature (extrinsic conduction). Thus, an organic conductor must have electron excess or an electron vacancy free to move in the whole solid. Valence and conduction bands are respectively the HOMO and LUMO of the molecule. But more important is to have a little gap between two bands; otherwise we make an insulator. In the Charge-Transfer complexes (CT) these conditions derive from HOMO of the donator and the LUMO of the acceptor.

When we talk about semiconductors we think to Silicon and Germanium; at room temperature some electrons can jump from valence band (VB) to conduction band (CB), realizing an Intrinsic Semiconductor. When we dope the Silicon or Germanium with appropriate atoms such as Boron we take place electron vacancy in the lattice of the Silicon or in other manner with Arsenic we take place electron excess. In both type of doping we have obtained an extrinsic conductor.
Organic compounds conduct electricity if inside there are present excess or vacancy of electrons free to move through the whole solid. In order to obtain organic conductors we must have a small energy gap between valence band and conduction band respectively derived from HOMO and LUMO of the organic molecules. In Charge Transfer Complexes (CT) these states derive from HOMO and LUMO of respectively of electron-donor-species and electron-acceptor-species:
A + D
® A.-D.+(Angew. Chem. I. E. ,1981,20,361) - (Acc. Chem. Res. 1974,7,232)
Metal state can be obtained in certain organic salts such as charge transfer complexes. In order to obtain them it’s necessary to keep to a few requirements:
FEATURES of the ACCEPTOR
The most suitable of Acceptor is:
TetraCyano-p-Quinodimethane, (TCNQ).
FEATURES of the DONOR
The most suitable of Donor is:
TetraThioFulvalene, (TTF).
Notice that the most suitable of CT-salts is: TTF-TCNQ !!!!!
In the 1960 was reported that some charge transfer complexes (CT) conduct electricity. The formation of these CT-salts requires two chemical "entities" that we call: DONOR (A) and ACCEPTOR (A). If we go to mix the DONOR with ACCEPTOR we take a place of a charge transfer from DONOR to ACCEPTOR:
A + D
® [A.-D.+]The chemical entities must have the precise features as follow:
Features of the chemical entities |
||
|
electron-donor Donor ( p -Donors) |
electron-rich-molecules Low Ionization Potential p -excessive-heterocycles |
it will form Cation or Radical-Cation |
|
electron-acceptor Acceptor ( p -Acceptors) |
electron-poor-molecules High Electron Affinity p -deficient-heterocycles |
it will form Anion or Radical-Anion |
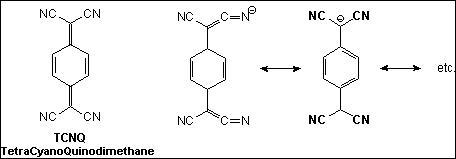
In 1960 was synthesized the first Acceptor such as
TetraCyano-p-Quinodimethane , (TCNQ) that forms a series of CT-salts. But these CT-salts are conductors but with low conductivity. The formation of these crystalline complexes involves the transfer of a single electron by a reducer (Donors) to the TCNQ (Acceptor):D.S.Acker,R.J.Harder,W.R.Hertler,W.Mahler,L.R.Melby,R.E.Benson,W.E.Mochel,J.Am.Chem.Soc. 1960,82,6408
The TCNQ make conductor-salts with electron-transfer from the electron-donor (Metals or other reducer species) to it with formation of stable radical-anion: TCNQ.-.
The first series of these compounds was reported with formula: M+TCNQ.- (for example: Li+TCNQ.-) that it has been show large resistivity (104-1012 ohm cm), then low conductivity. While the second series of these compounds was reported with formula: M+TCNQ.-TCNQ (for example (C2H5)3NH+(TCNQ.-)(TCNQ)) that has been show most conductivity respect the first series.
The TetraThioFulvalene, (TTF), was already well-known until 1964 with its synthesis on 1970. The molecule has particular features for become a good Donor. In fact TTF is very interesting for its reactivity that comparable to an electron-rich-olefine that when react with an electron-poor-olefine take place the stable radical-cation.

E.Klingsberg , J.Am.Chem.Soc. 1964,86,5290
F.Wudl,G.M.Smith,E.J.Hufnagel , J.Am.Chem.Soc. 1970,1453
In 1973 was discovered the first CT-salts (1:1) formed by TTF and TCNQ with a maximum of conductivity 104 S/cm at T=59 K,(-214°C).

J.Ferraris,D.O.Cowan,V.Walatka,Jr., J.H.Perlstein,J.Am.Chem.Soc.1973,95,948
The Tetrathiofulvalene (TTF) Family
In the following years was made some manipulation of TTF to find a better electron-donor by two ways:
Substituting the sulfur atoms with other elements like Selenium and Tellurium, exploiting the heavy atom effect.

Substituting the ring with groups to stabilize cation-radical.


Elson,Kochi,Klabunde,Manzer,Parshall,Tebbe , J.Am.Chem.Soc. 1974,96,7376
The structure of TSeF is not so different on TTF but it allows the increase of polarization and overlap between the orbital with decrease of coulombic interactions.
The conductivity of TSeF-TCNQ is better then TTF-TCNQ and at temperature of 25 °C (198K) is equal to 800 ohm-1cm-1 while at 40 K the conductivity is 104 ohm-1cm-1.
Another modification of the TSeF is the next synthesis of (TMTSF), TetraMethylTetraSelenoFulvalene :

K.Bechgaard,D.O.Cowan,A.N.Bloch J.Chem.Soc.Chem.Commun. 1974,937
In 1981 was discovered the first Bechgaard Salt where the Anion is not TCNQ but an inorganic-ion such as ClO4- that was demonstrated be a good superconductor. In fact the (TMTSF)2ClO4 is a good conductor at temperature between 0.5-1.5 K and obtained by means of electrochemical oxidation:
2nTMTSF + nClO4- + ne-
® [(TMTSF)2ClO4]n
McCullough,Gaik,Kok,Knud,Lerstrup,Cowan , J.Am.Chem.Soc. 1987,109,4115
If we replace the sulfur atom with Tellurium we have the TetraTelluraFulvalene
; (TTeF) where is important the propriety of Tellurium that can be undergo polarization and reduce the Coulombic interactions with more stability of radical-cation in CT-salt formed. The expansion of the p-orbital of Tellurium allow a good overlap between next other orbital.Bis-(EthilenDiThio)-TetraThioFulvalene
With the second manner to modify the TTF we arrive to obtain a complicate molecules with four atoms to increase the stabilization of radical-ions:

Bis-(EthilenDiOxi)-TetraThioFulvalene

T.Suzuki,H.Yamochi,G.Srdanov,K.Hinkelmann,F.Wudl,J.Am.Chem.Soc.1989,111,3108.
In 1983 has been thought that is very useful insert a space-linker between two TTF for increasing the delocalizzation. In 1991 was synthesized the analogs TTF with space-linker:
(Symmetric)

T.K.Hansen,M.V.Laksmikantham,M.P.Cava,R.M.Metzger,j.Becher,J.Org.Chem.,1991,56,2720
A.J.Moore,M.R.Bryce,D.J.Ando,M.B.Hursthouse,J.Chem.Soc.Commun.1991,320
(Asymmetric)

T.K.Hansen,M.V.Laksmikantham,M.P.Cava,R.M.Metzger,j.Becher,J.Org.Chem.,1991,56,2720
A.J.Moore,M.R.Bryce,D.J.Ando,M.B.Hursthouse,J.Chem.Soc.Commun.1991,320
Chem. Soc. Rev. 1991,20,355-390